Biofoundries: a revolution for research and global economy
In the rapidly evolving field of biotechnology, biofoundries represent a transformative shift in how research and development are conducted. These state-of-the-art facilities combine automation, synthetic biology, and advanced computational tools to accelerate the design, building, testing, and learning (DBTL) cycle of biological systems. Biofoundries enable the integration of complex biological processes into streamlined, automated workflows, leading to unprecedented efficiency and accuracy in the development of engineered organisms and biological systems. As we stand on the brink of a new era in biotechnological innovation, understanding the role and potential of biofoundries is essential for grasping the future of science and industry.
What are biofoundries?
Biofoundries are specialized facilities designed to streamline the development of biological systems using high-throughput, automated technologies. They integrate various processes—such as DNA synthesis, gene editing, strain engineering, and metabolic pathway optimization—into a seamless workflow, enabling researchers to rapidly prototype and iterate biological constructs. The concept of a biofoundry is analogous to traditional manufacturing foundries, where raw materials are processed and transformed into finished products. However, in a biofoundry, the “raw materials” are biological parts, such as genes, proteins, and metabolic pathways, and the “finished products” are engineered organisms or biological systems with specific, desirable traits.

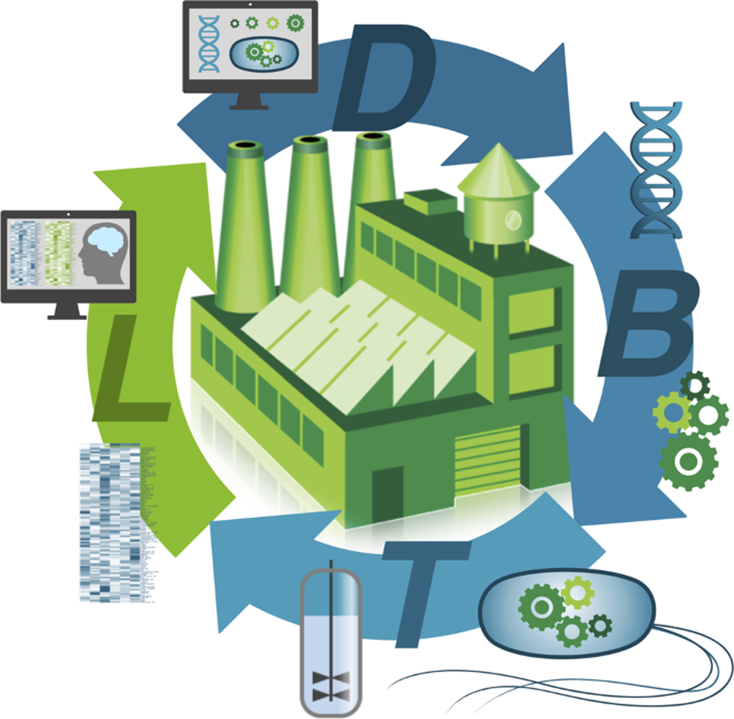
At the core of a biofoundry lies its ability to execute the DBTL cycle efficiently. This cycle consists of:
- Design: Using computational tools to design genetic circuits or metabolic pathways.
- Build: Constructing the designed biological components through automated synthesis and assembly techniques.
- Test: Evaluating the performance of the engineered systems through high-throughput screening.
- Learn: Analyzing data to refine designs and improve subsequent iterations.
This integrated approach significantly reduces the time and cost associated with biotechnological research, paving the way for rapid innovation in areas such as synthetic biology, metabolic engineering, and drug discovery. By automating these processes, biofoundries enhance reproducibility, scalability, and standardization, making complex biological engineering projects more feasible and efficient.
Applications of Biofoundries in Health Research
In recent years, biofoundries have made significant contributions to health research, particularly in the areas of vaccine development, personalized medicine, and the discovery of new therapeutic compounds. One of the most prominent examples of biofoundries’ impact on health research is their role in the rapid development of COVID-19 vaccines. The pandemic underscored the need for swift and scalable solutions to global health crises, and biofoundries played a pivotal role in meeting this challenge.
For instance, the COVID-19 mRNA vaccine relied heavily on synthetic biology techniques facilitated by biofoundries to quickly design and produce the mRNA constructs used in the vaccine. This rapid response was made possible by the automated workflows and high-throughput capabilities of biofoundries, which allowed for the quick scaling of vaccine production and testing (Crone et al., 2020, Nature Communications; Kitney et al., 2021, Trends in Biotechnology). The success of this approach has demonstrated the potential of biofoundries to revolutionize vaccine development and respond to future pandemics more effectively.
Biofoundries have also played a critical role in the discovery of new antibiotics, addressing the growing threat of antibiotic-resistant bacteria. The rise of antibiotic resistance is a major global health concern, and biofoundries have been instrumental in the search for new antimicrobial compounds. In recent years, researchers used a biofoundry to screen thousands of natural product extracts for antibiotic activity (Ayikpoe et al., 2022, Nature communications; Yuan et al., 2022, Nature Catalysis). The automated systems enabled the rapid identification of several promising candidates, which are now being further developed as potential new antibiotics. This capability highlights the importance of biofoundries in addressing one of the most pressing challenges in modern medicine.
In the field of personalized medicine, biofoundries have the potential to be utilized to develop patient-specific treatments, particularly in oncology and metabolic diseases. The steadfast advance of synthetic biology has empowered scientists to leverage genetically engineered cells, which act as therapeutic agents rather than traditional small molecules or biologics (Cubillos-Ruiz et al., 2021, Nature Reviews Drug Discovery). These engineered cells, programmed with synthetic gene circuits, are capable of precisely controlling the localization, timing, and dosage of therapeutic activities in response to specific disease biomarkers. This technology offers a revolutionary approach to treatment, particularly in cancer and genetic disorders, as it provides a level of flexibility, specificity, and predictability that conventional therapies lack. Such advancements underscore the potential of biofoundries to accelerate the development of these cutting-edge, cell-based therapies, which are rapidly becoming a cornerstone in the fight against a wide array of human diseases.

Impact of Biofoundries on Research Workflow
The integration of biofoundries into the research workflow is poised to transform how scientific research is conducted. Traditional research methods in biotechnology often involve labor-intensive and time-consuming processes, with significant variability in results due to the manual nature of many experimental procedures. Biofoundries address these challenges by automating key aspects of the research process, from the design and construction of biological systems to their testing and optimization.
One of the most significant impacts of biofoundries on research workflow is the ability to perform high-throughput experiments with minimal human intervention. By utilizing robotics and liquid handling systems, biofoundries can conduct thousands of experiments simultaneously, drastically increasing the speed of data generation. This capability is particularly valuable in fields like synthetic biology and drug discovery, where the ability to rapidly test and iterate on designs is critical to success.
In addition to increasing throughput, biofoundries also enhance the reproducibility of research. The automation of experimental procedures reduces the variability introduced by human error, leading to more consistent and reliable results. This improvement in reproducibility is crucial for the validation of scientific findings and for the development of products that must meet stringent regulatory standards. Furthermore, biofoundries facilitate the integration of computational tools into the research process. Machine learning algorithms can be used to analyze the vast amounts of data generated by high-throughput experiments, identifying patterns and guiding the design of new experiments. This combination of automation and data analysis creates a feedback loop where the results of one experiment inform the next, accelerating the overall research process (Chao et al., 2017, Metabolic Engineering).
Biofoundries also enable more complex and ambitious research projects by providing a platform for the integration of multiple disciplines. For example, a biofoundry might combine expertise in synthetic biology, bioinformatics, and chemical engineering to tackle a challenge like producing bio-based materials. This interdisciplinary approach is increasingly important in modern research, where solving complex problems often requires the collaboration of experts from different fields. By facilitating these collaborations, biofoundries are helping to push the boundaries of what is possible in biotechnology and related fields.
Biofoundries and the Global Economy in the Fourth Industrial Revolution
The advent of biofoundries represents a significant development in the context of the Fourth Industrial Revolution, characterized by the fusion of biological, physical, and digital technologies. Biofoundries are at the forefront of this transformation, driving innovation in biotechnology and contributing to the global economy in several ways.
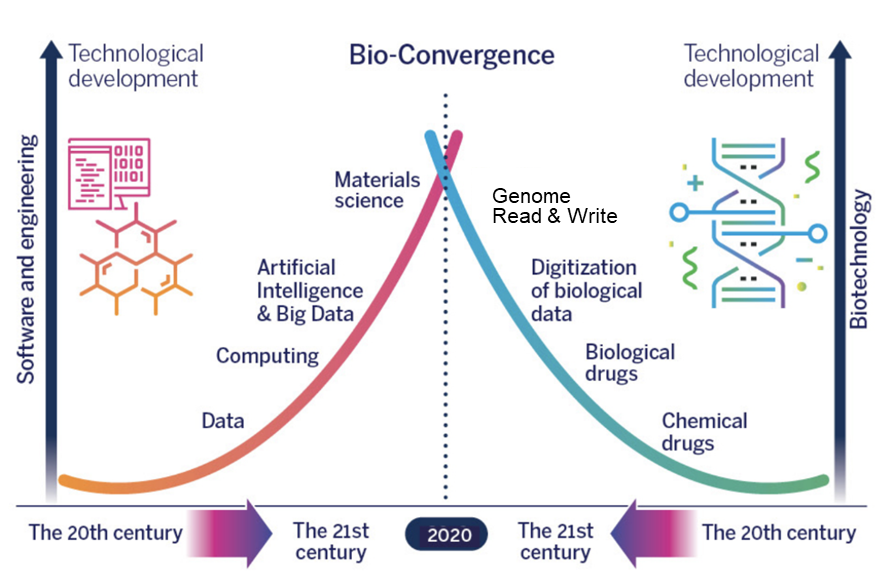
Firstly, biofoundries are expected to play a crucial role in the bioeconomy, which is projected to grow substantially in the coming decades. By enabling the rapid development and production of bio-based products, biofoundries are helping to reduce reliance on fossil fuels and promote the use of renewable resources. This shift has the potential to create new industries and jobs, particularly in areas such as bio-based chemicals, sustainable agriculture, and renewable energy. To illustrate, consider the surge in startups within the synthetic biology sector, which attracted 9 billion euros in the first half of 2021—more than twice the amount of venture capital investments from the previous year. Companies like Amyris, Genomatica, Ginkgo Bioworks, Zymergen, and OpenTrons are now valued at over $1 billion each. Additionally, various indicators point to a global influx of several trillion dollars into the bioeconomy.
Moreover, the automation and digitization of biological research enabled by biofoundries are likely to lead to significant cost reductions and efficiency gains in various industries. For example, in pharmaceuticals, the ability to rapidly prototype and test new drug candidates could reduce the time and cost of bringing new drugs to market. This efficiency is not only beneficial for the companies involved but also for the global economy, as it could lead to more affordable medicines and faster responses to health crises. Additionally, the potential for biofoundries to democratize access to advanced biotechnology is significant, particularly in developing countries. By lowering the barriers to entry for biological research and production, biofoundries can help to level the playing field and enable more countries to participate in the bioeconomy. This democratization could lead to more diverse innovation, with different regions contributing unique solutions to global challenges (Vickers and Freemont, 2022, Nature Communications).
Examples of Existing Biofoundries and Their Achievements
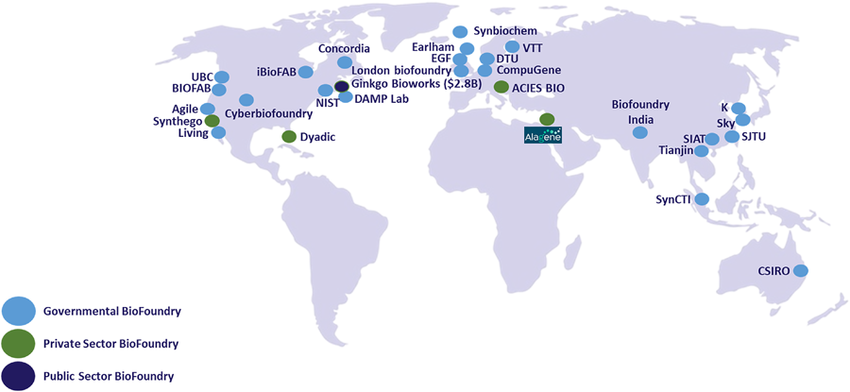
Numerous biofoundries around the world have already made significant contributions to biotechnology and related fields. These facilities exemplify the potential of biofoundries to drive innovation and address global challenges.
One of the most prominent biofoundries is the Joint BioEnergy Institute (JBEI) in the United States, which focuses on developing advanced biofuels. JBEI has made significant strides in the development of clean, sustainable biofuels and bioproducts. By leveraging advanced techniques in molecular biology, chemical engineering, and genetics, JBEI’s interdisciplinary team has engineered microbes capable of converting biomass sugars into energy-rich biofuels, such as gasoline, diesel, and jet fuel. These advanced biofuels are designed to seamlessly integrate with existing engines and infrastructure without compromising performance. JBEI’s work also focuses on optimizing bioenergy crops, or “feedstocks,” to improve their conversion efficiency and environmental resilience. The institute is developing more effective methods for breaking down lignocellulosic biomass into fermentable sugars, which are crucial for biofuel production. Additionally, JBEI is conducting field trials to assess the environmental and economic impacts of these biofuels, aiming to meet significant portions of the nation’s energy needs sustainably.
In the United Kingdom, the Imperial College London Biofoundry has made significant strides in synthetic biology and biomanufacturing. This facility has developed a platform for the rapid prototyping of genetic circuits, allowing for the assembly and testing of complex genetic constructs in a matter of days (Hoose et al, 2023, Nature Reviews Chemistry). The London Biofoundry partners with companies to deliver innovative biological solutions using state-of-the-art technologies. By collaborating closely with industry teams, the Biofoundry offers expertise in directed evolution, strain engineering, and automated workflows, facilitating the development and production of novel biological products. Their capabilities include advanced DNA synthesis, automated cloning, high-throughput screening, and large-scale bioreactor production. Additionally, the Biofoundry provides comprehensive analytical support through techniques like flow cytometry, next-generation sequencing, and live-cell imaging. These services enable companies to accelerate their R&D processes and bring cutting-edge biotechnological advancements to market more efficiently.
Another example is the Edinburgh Genome Foundry (EGF) in Scotland. It specializes in the modular assembly of DNA constructs using advanced automated robotic platforms, offering extensive support for both academic and industrial research projects in creating personalized therapies. EGF is at the forefront of synthetic biology, facilitating projects that range from programming stem cells for personalized medicine to developing viral vaccines and gene therapies. Their capabilities include high-throughput DNA assembly for various organisms, such as bacteria, yeast, and mammalian cells, and they provide a range of cell phenotyping assays, including qPCR and high-throughput micro-fermentation. A standout tool at EGF is the Berkeley Lights Beacon, which enables precise single-cell cloning and deep cell phenotyping. This platform, alongside EGF’s automation solutions, accelerates research with reliable, cost-effective execution of complex tasks. EGF also provides industry-grade software for DNA design and lab automation, enhancing the efficiency of synthetic biology projects.
The Australian Genome Foundry offers advanced capabilities in strain design and engineering, particularly for large-scale genetic modifications in yeast and bacteria. Their expertise includes creating combinatorial genetic libraries and screening strain libraries generated through random chemical mutagenesis. They utilize high-throughput fermentation technologies, enabling the growth and analysis of hundreds to thousands of strains daily, with controlled fermentation options available. The Foundry also provides sophisticated analytical chemistry services, including metabolite and protein screening, as well as flow cytometry, cell sorting, and qPCR for gene expression profiling. Additionally, they offer comprehensive project management, integrating the entire Design, Build, Test, and Learn cycle.
All the aforementioned biofoundries are members of the Global Biofoundries Alliance (GBA), an initiative which fosters communication among skilled professionals in a collaborative, non-competitive environment to accelerate technological advancements. Comprising 35 biofoundries as of the writing of this article, the alliance operates with a commitment to global inclusivity and public interest. The GBA’s mission is to advance synthetic biology technologies for the benefit of humanity and the planet, with a focus on techno-economic projections as a significant, though not exclusive, motivating factor (Hillson et al., 2019, Nature Communications). The Paris Bioconvergence Biofoundry, co-financed by the DIM BioConvS, is currently working to join the alliance. >>See all current members of the GBA
Why Ile-de-France Will Benefit from Having an Academic Biofoundry
The current global landscape for biofoundries is one of rapid growth and increasing interest from both the public and private sectors. Countries around the world are recognizing the strategic importance of biofoundries in driving innovation and maintaining competitiveness in the global bioeconomy. The United States, the United Kingdom, and China are among the leaders in this field, with significant investments in biofoundry infrastructure and research.
For France, the development of an academic biofoundry in the Ile-de-France region could have significant benefits. Ile-de-France is already a hub for research and innovation, with a concentration of leading universities, research institutions, and biotechnology companies. Establishing a biofoundry in this region would further enhance its status as a center of excellence in biotechnology and synthetic biology.
A biofoundry in Ile-de-France would provide French researchers with access to state-of-the-art facilities and tools, enabling them to compete on the global stage. It would also attract international talent and investment, further boosting the region’s economy and innovation ecosystem. Moreover, by fostering collaboration between academia and industry, a biofoundry could help to bridge the gap between research and commercialization, accelerating the development of new products and technologies.
It is in this context that the Biofonderie Paris Bioconvergence project is developing. A distributed biofoundry with DNA synthesis, scaling up, cell-free, microorganisms strains and mammalian cells facilities, this ambitious project is being carried out by the leading experts in Synthetic Biology in France and has received generous funding from the Région Ile-de-France, as well as a considerable co-funding from all participating institutions.
In the context of the Fourth Industrial Revolution, France’s investment in biofoundries will position the country as a leader in the bioeconomy. As global challenges such as climate change and pandemics continue to shape the future, having a strong biofoundry infrastructure will be crucial for addressing these issues through biotechnology. Furthermore, by reducing reliance on fossil fuels and promoting the use of renewable resources, biofoundries can contribute to France’s sustainability goals and its transition to a greener economy in line with the France 2030 policy.
References
Ayikpoe, R.S., Shi, C., Battiste, A.J., Eslami, S.M., Ramesh, S., Simon, M.A., Bothwell, I.R., Lee, H., Rice, A.J., Ren, H., Tian, Q., Harris, L.A., Sarksian, R., Zhu, L., Frerk, A.M., Precord, T.W., van der Donk, W.A., Mitchell, D.A., Zhao, H., 2022. A scalable platform to discover antimicrobials of ribosomal origin. Nat Commun 13, 6135. https://doi.org/10.1038/s41467-022-33890-w
Crone, M.A., Priestman, M., Ciechonska, M., Jensen, K., Sharp, D.J., Anand, A., Randell, P., Storch, M., Freemont, P.S., 2020. A role for Biofoundries in rapid development and validation of automated SARS-CoV-2 clinical diagnostics. Nat Commun 11, 4464. https://doi.org/10.1038/s41467-020-18130-3
Cubillos-Ruiz, A., Guo, T., Sokolovska, A., Miller, P.F., Collins, J.J., Lu, T.K., Lora, J.M., 2021. Engineering living therapeutics with synthetic biology. Nat Rev Drug Discov 20, 941–960. https://doi.org/10.1038/s41573-021-00285-3
Hillson, N., Caddick, M., Cai, Y., Carrasco, J.A., Chang, M.W., Curach, N.C., Bell, D.J., Le Feuvre, R., Friedman, D.C., Fu, X., Gold, N.D., Herrgård, M.J., Holowko, M.B., Johnson, J.R., Johnson, R.A., Keasling, J.D., Kitney, R.I., Kondo, A., Liu, C., Martin, V.J.J., Menolascina, F., Ogino, C., Patron, N.J., Pavan, M., Poh, C.L., Pretorius, I.S., Rosser, S.J., Scrutton, N.S., Storch, M., Tekotte, H., Travnik, E., Vickers, C.E., Yew, W.S., Yuan, Y., Zhao, H., Freemont, P.S., 2019. Building a global alliance of biofoundries. Nat Commun 10, 2040. https://doi.org/10.1038/s41467-019-10079-2
Hoose, A., Vellacott, R., Storch, M., Freemont, P.S., Ryadnov, M.G., 2023. DNA synthesis technologies to close the gene writing gap. Nat Rev Chem 7, 144–161. https://doi.org/10.1038/s41570-022-00456-9
Kitney, R.I., Bell, J., Philp, J., 2021. Build a Sustainable Vaccines Industry with Synthetic Biology. Trends Biotechnol 39, 866–874. https://doi.org/10.1016/j.tibtech.2020.12.006
Vickers, C.E., Freemont, P.S., 2022. Pandemic preparedness: synthetic biology and publicly funded biofoundries can rapidly accelerate response time. Nat Commun 13, 453. https://doi.org/10.1038/s41467-022-28103-3
Yuan, Y., Cheng, S., Bian, G., Yan, P., Ma, Z., Dai, W., Chen, R., Fu, S., Huang, H., Chi, H., Cai, Y., Deng, Z., Liu, T., 2022. Efficient exploration of terpenoid biosynthetic gene clusters in filamentous fungi. Nat Catal 5, 277–287. https://doi.org/10.1038/s41929-022-00762-x
Disclaimer: This article has been redacted with the assistance of artificial intelligence to ensure clarity and coherence. Please note that while the editing process involved AI tools, all sources and data referenced are authentic and have been rigorously verified. The content reflects accurate and up-to-date scientific information.
Action financée par la Région Île-de-France
Follow us on LinkedIn
Join our LinkedIn group
Contact: contact@bioconvs.org
Consult our data protection policy
Address:
DIM BioConvS
Faculté des Sciences - Université Paris Cité
5 rue Thomas Mann 75013 Paris


